“600 000 people in the UK suffer from IBD,” says Poletti, “and many drugs don’t work for them, so it’s important to understand what’s going on.”
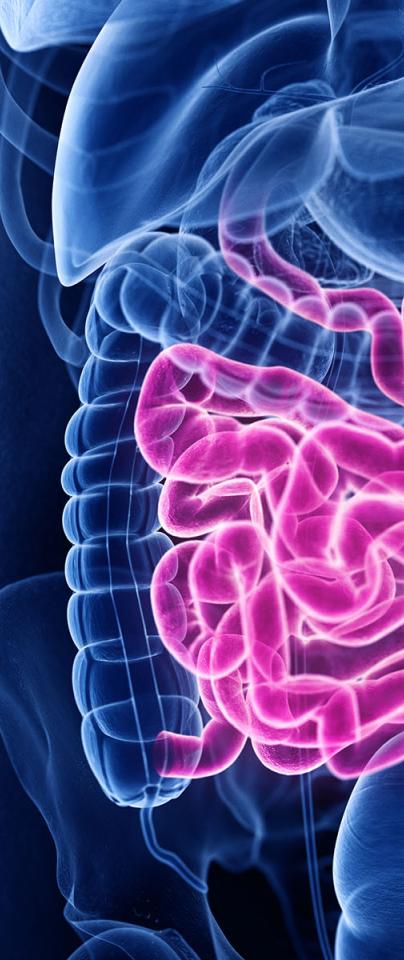
IBD - or Inflammatory Bowel Disease - describes a range of symptoms characterised by inflammation of the gut (see info box below), which are broadly grouped into two main conditions: Crohn’s disease (CD) and ulcerative colitis (UC).
“Crohn’s affects the top of the large intestine - the colon - and the last part of the small intestine. By comparison, people with ulcerative colitis have inflammation at the end of their colon - the rectum - and this usually progresses upwards,” says Hautefort. “Certain new drugs can reduce the inflammation associated with IBD but, for the majority of patients, this effect soon subsides.
“Unfortunately, that means there’s no cure for any of these lifelong chronic diseases - they’re multi-factoral, very complex, and differ between patients even if they display the same symptoms. It’s absolutely essential we understand why.”
One of the key factors being investigated is our food. “With western lifestyles and diets, general gut health is declining and a lot of immune-related diseases are increasing, including IBD and eczema,” explains Treveil, who is coming to the end of a successful PhD, with a first author publication and an award for best conference talk (among a whopping field of 1100) already to her name.
“To get to the bottom of this, you have to go to the basics: the interactions in the gut with bacteria, our immune system, and food. The list of things that can affect your gut is endless.”